Dr. M. Shiva Prasad
July 24, 2023 2024-03-27 10:30
|
| ||||
|
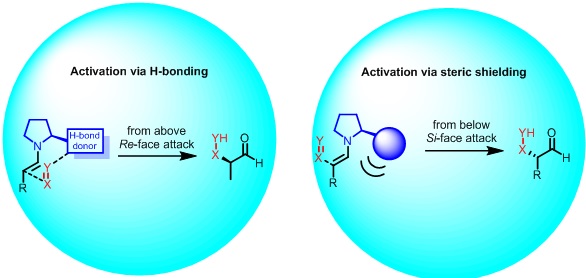
Research Highlights : MSP research group focuses on two major research areas:Organocatalysis: Synthesis of chiral molecules primarily relies on catalysis. Organocatalysis mainly follows the iminium/enamine mechanism. MSP group focuses on developing synthetic methodologies via organocatalysis that could induce chirality in two primary modes. 1. Hydrogen –bond- directing catalysis MSP group research interests center around the asymmetric synthesis of small natural products and natural product-like molecules by exploiting catalysis. His research group also focuses on developing new synthetic methods to generate morphologically and functionally complex small chiral organic molecules with biological activity.
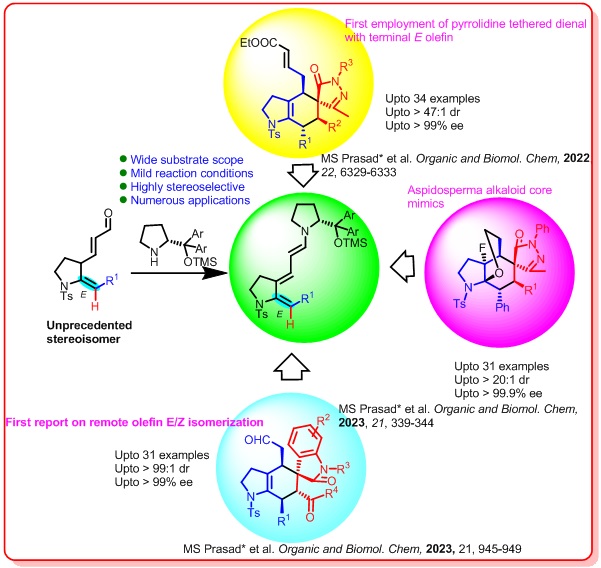
Recently MSP research group focussed on developing a robust and sustainable approach for constructing functionally rich optically pure heterocyclic frameworks via [4+2]-addition of in situ generated trienamine from a specially designed 2-(E)-benzylidine-3-pyrrolidinyl acraldehyde as a synthon.
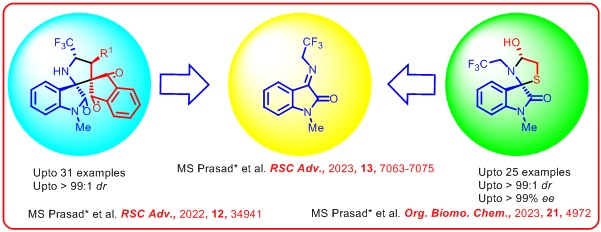
MSP research group is also interested in other aminocatalytic cascade reactions by employing ketiminesas a potential synthon for constructing biologically potential spiroheterocycles.
Recent Publications : 1. Madavi S. Prasad,*Aman Kumar Jha and, Sankar Bharani, “Stereoselective Synthesis of Functionally Rich Spirooctahydroquinoline Oxindoles via Enynamide Cycloisomerisation-/[4+2]-Addition Sequence” Adv. Synth.Catal.,2024, xx, 202400162. https://doi.org/10.1002/adsc.202400162 2. Madavi S. Prasad,*M. Ramesh, Aman Kumar Jha, Sankar Bharani, Souvik Saha, ThoudamBedanta, Ponnada Chandrasekhar,and M. Sarasija, “Amine Catalyzed Remote [4+2] Annulation of Indole Tethered Enal and Oxindole Olefin to Access Optically Pure HydrocarbazoleSpirooxindoleScaffolds Asian J. Org. Chem. 2024, xx, e202400061. https://doi.org/10.1002/ajoc.202400061 3.Madavi S. Prasad,* Sankar Bharani, Aman Kumar Jha, Sugali Chetan Naik, Murugesan Sivaprakash, and L. Raju Chowhan, “Enantioselective synthesis of octahydrofuranoindole core of aspidosperma alkaloids via Diels Alder/reduction/fluoroetherification reaction sequence” Chem. Asian J.,2023, 18, e202300419.https://doi.org/10.1002/asia.202300419 4. Madavi S. Prasad,* Sankar Bharani, Murugesan Sivaprakash, Prabha Vadivelu, D. Siva Sundara Kumar and L. Raju Chowhan, “N-2,2,2-trifluoroethylisatinketimine as an unprecedented 1,2-dipolarophile for [3+2] addition to access optically pure spiro- thiazolidine oxindoles” Org. Biomol. Chem.,2023, 21, 4972-4976. https://doi.org/10.1039/D3OB00685A 5. Biplob Borah, Murugesan Sivaprakash, Samrita Sharma, Madavi S. Prasad*, and, L. Raju Chowhan, “Blossoming of polyenamine catalysis in asymmetric synthesis: Scope and future applications” Chem. Asian J.,2023, 18, e202300370. https://doi.org/10.1002/asia.202300370 6. Madavi S. Prasad*, Murugesan Sivaprakash, and Sankar Bharani, Trienamine catalyzed unprecedented remote olefin E/Z isomerization/[4+2]-cycloaddition reaction to access spirooxindole hexahydroindoles. Org. Biomol. Chem.,2023, 21, 945 – 949. https://doi.org/10.1039/D2OB02228A 7. Madavi S. Prasad*, and Murugesan Sivaprakash, Asymmetric synthesis of the perhydroepoxyethanoindole core via sequential [4 + 2]-addition/ reduction/fluoroannulation reactions. Org. Biomol. Chem., 2023, 21, 339-344. https://doi.org/10.1039/D2OB02058K 8. Biplob Borah, Murugesan Sivaprakash, Madavi S. Prasad*, and L. Raju Chowhan, Visible-light-induced Organophotocatalytic and Singlet Oxygen-initiated Domino construction of 1,4-dihydropyridines, quaternary centered C-3 functionalized Spiro[indoline-3,4'-pyridines] and C-11 functionalized Spiro[indeno-[1,2b]quinoxalines-11,4'-pyridines]. Org. Biomol. Chem., 2023, 21, 1518-1530. https://doi.org/10.1039/D3OB00043E 9. Biplob Borah, ..., Madavi S. Prasad*, and L. Raju Chowhan*, Stereoselective synthesis of CF3-containing spirocyclic-oxindoles using N-2,2,2-trifluoroethylisatin ketimines: An update RSC Adv.,2023, 13, 7063-7075.https://doi.org/10.1039/D3RA00017F |
|||||